Copper Current-Collector Dissolution
Application ID: 126981
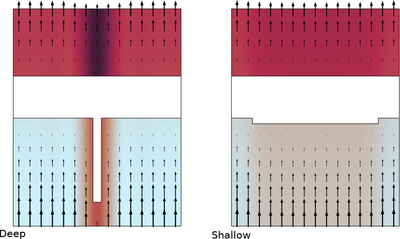
The copper current collector on negative graphite electrodes in lithium-ion batteries have been seen to dissolve at over discharge. This can be a safety concern as the dissolution damages the current collector irreversibly and dissolved copper ions can redeposit and form dendrites. Batteries are usually operated within specified voltage limits, either on cell or pack level, in order to avoid over discharge. However, over discharge may still occur, either in a pack due to charge imbalances between the individual cells, or, as we will partly investigate in this tutorial, due to local heterogeneities in the electrodes within a single battery cell.
This tutorial investigates the copper current-collector dissolution during overdischarge from an electrode with an inhomogeneous graphite electrode material coating thickness. The cuprous ion concentration, as well as the redeposited copper distribution in the cell, are predicted.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Specification Chart and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.
