Development of Electrochemical Methods for Differentiation of the Catecholamine Neurotransmitters
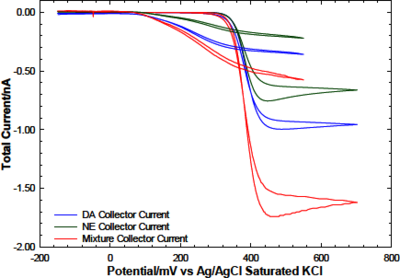
Neurological disorders afflict tens of millions in the US. We are developing technology to advance capabilities for monitoring the neurotransmitters, dopamine, norepinephrine, and epinephrine (the catecholamines), to facilitate direct observations of their mutual interactions in brain function. Imbalances in their concentrations are often linked to neurological disorders [1]. It is critical to combine modeling with experiments to develop optimized technology that will enable new discoveries of causes, diagnosis, prevention, and cure of neurological illnesses. The structural similarities have made real time, in vivo quantification and differentiation of the catecholamines difficult. Redox cycling – generation-collection – attempts to overcome this problem by using individually-addressable microelectrode arrays that measure current from oxidation and reduction of the catecholamines and are mounted on probes as thin as a hair for insertion into tissue with minimal damage. One set of microelectrodes serves as “generators” to oxidize the catecholamines to orthoquinones. Another set of microelectrodes serve as “collectors” to re-reduce the orthoquinones back to the catechol form. The magnitudes of the currents at the generator-collector electrodes are proportional to the concentrations of the corresponding species. These measurements simultaneously serve as a characterization of the mass transfer and extent of chemical reactions that occur in solution while the species travel between generators and collectors, which can enhance or diminish the concentrations during that time. Consequently, many parameters influence the generation-collection outcomes at electrode arrays, e.g. the device’s physical properties, electrode geometry, size, and spacing, and the applied potential at the electrodes. An in silico model based on prior experimental generation-collection studies is being advanced and focuses on differentiating dopamine from norepinephrine and epinephrine by measuring the current at the collectors at various distances from the generators [2]. This approach takes advantage of the different kinetics of the reaction mechanisms for each type of catecholamine after they oxidize at the generators. It has been established that catecholamines undergo an ECC’ mechanism: a two-electron oxidation (E) (at the generators) to the orthoquinone, followed by a 1,4-addition and results in cyclization (C) to the leucoaminochrome with different rate constants: epinephrine>norepinephrine>dopamine. A following fast-bimolecular reaction (C’) involves a leucoaminochrome and another orthoquinone, producing an aminochrome and the catechol form that oxidizes again at the generators [3, 4]. With sufficiently close collectors, the orthoquinones survive the trip from the generators, before other chemical mechanisms take place, producing a measurable collector current. The orthoquinones of dopamine and norepinephrine can be detected, but epinephrine’s cannot when collectors are ≥4 µm away from generators. Further differentiation occurs at distances ≥20 µm, when collectors cannot detect norepinephrine’s orthoquinone, but still exhibit a current from dopamine’s orthoquinone [2]. COMSOL Multiphysics® is being used to optimize parameters and study concentration profiles, interactions, redox cycling of catecholamines and to facilitate the interpretation of experimental results and development of probe designs capable of detecting in vivo concentrations of these neurotransmitters. The model includes the electrochemical and mathematics modules of COMSOL Server.TM The electrochemical module studies the generation-collection approach. The mathematics module solves a set of twelve partial differential equations describing the transport phenomena of the catecholamines.
- Arnsten, A. F. T.; Pliszka, S. R., Catecholamine Influences on Prefrontal Cortical Function: Relevance to Treatment of Attention Deficit Hyperactivity Disorder and Related Disorders. Pharmacology, biochemistry, and behavior 2011, 99 (2), 211-216.
- Hu, M.; Fritsch, I., Application of Electrochemical Redox Cycling: Toward Differentiation of Dopamine and Norepinephrine. Analytical Chemistry 2016, 88 (11), 5574-5578.
- Ciolkowski, E. L.; Cooper, B. R.; Jankowski, J. A.; Jorgenson, J. W.; Wightman, R. M., Direct observation of epinephrine and norepinephrine cosecretion from individual adrenal medullary chromaffin cells. Journal of the American Chemical Society 1992, 114 (8), 2815-2821.
- Hawley, M. D.; Tatawawadi, S. V.; Piekarski, S.; Adams, R. N., Electrochemical Studies of the Oxidation Pathways of Catecholamines. Journal of the American Chemical Society 1967, 89 (2), 447-450.
Download
- abrego tello_presentation.pdf - 0.89MB
- abrego tello_poster.pdf - 2.2MB
- abrego tello_abstract.pdf - 0.02MB
