Lithium-Ion Battery Aging with Varying Solvent Composition Effects
Application ID: 140241
The liquid electrolyte in lithium-ion batteries (LIBs) typically consists of a lithium salt, such as LiPF6, dissolved in one or several solvents.
Commercial LIBs commonly employ a mix of multiple hydrocarbon-based solvents along with additional additives. In an electrolyte consisting of multiple solvents, the local electrolyte transport properties within the separator and the electrodes, such as conductivity, may depend not only on the salt concentration but also on the ratio of the different solvents.
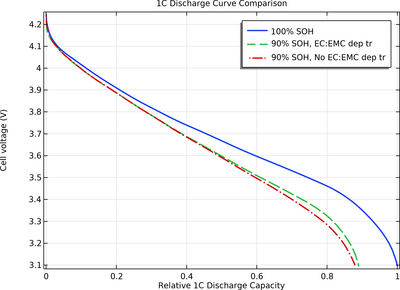
As the battery ages, parasitic reactions may predominantly consume one of the solvents, resulting in a varying ratio of the different solvents over time. This change can, in turn, alter how the electrolyte transport properties are influenced by the local salt concentration in the cell throughout the battery's lifetime.
This tutorial model explores how the consumption of one of the solvents in an aging battery cell impacts its 1C discharge capacity.
To learn more about this model, see our accompanying blog post "The Effects of Varying Solvent Compositions on Lithium-Ion Battery Aging".
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Specification Chart and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.
